0
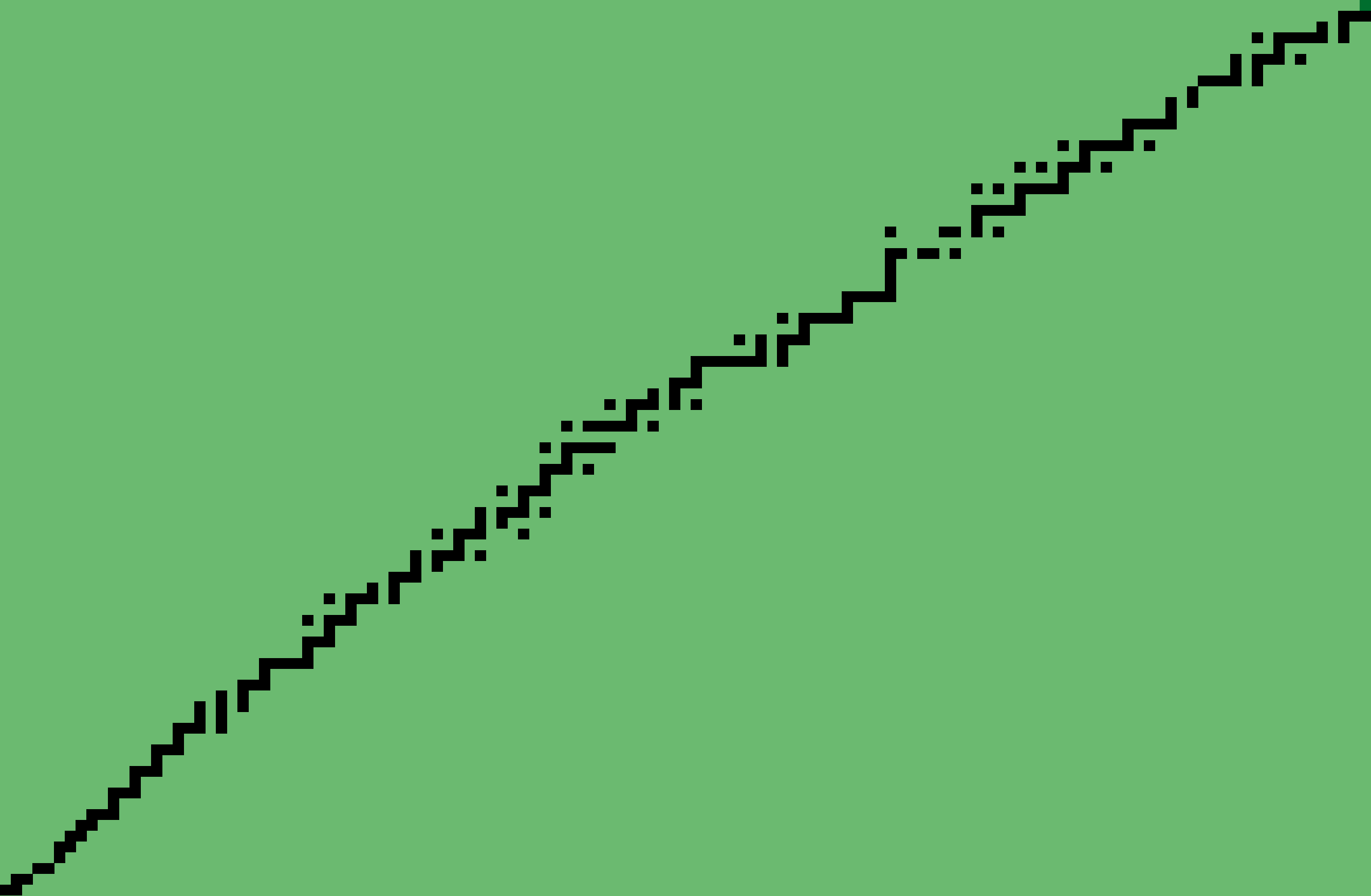
 The Stable Nuclides
The Stable Nuclides
22 Apr 2017 16:29 by
volodymyr.velyky
Proton number increases upward, neutron number increases rightward.
Thus Hydrogen-1 is at the lower left, and Bismuth-209* at the upper right
(83 protons, 126 neutrons). And that's all there are!
An alien civilization would not have our names for the elements,
might not have our numbering system,
but if they're developed enough for spacefaring, they will have this chart.
Plus, it's pretty.
* Bismuth-209 is a special case. It is unstable (alpha-decay) but its half-life is orders of magnitude longer than other unstable nuclides, and for that matter well longer than the age of the universe. I might decide to leave it out on the technicality.